eCQM Meaningful Use Criteria for ODs
Reporting on eCQMs is part of meeting the Meaningful Use criteria for ODs participating in the incentive program. Whether you are just starting out with Stage 1 to avoid further penalties or moving on with Stage 2, it can still get complicated. So we’re here to give you some basics and resources to get started with eCQMs.
eCQM Reporting for Meaningful Use Criteria for ODs
To start, eCQMs stand for electronic clinical quality measures. eCQM’s are reports that measure and track the quality of health care services being provided. These reports are created with a wide variety of data that is associated with a practice’s ability to deliver high-quality care.
eCQM Reporting Time
When we said that reporting on eCQMs is a necessary part of completing the Meaningful Use Program, we don’t mean that they have to be done at the same time. The reporting periods do not have to align. The reporting period for CQMs is 90 days for the first year. And after the first year, the reporting period is 1 quarter of the calendar year, and it doesn’t have to align with your incentive program 90 day reporting period.
NQS Domains

For 2015, reporting on eCQMs requires 9 measurements from across 3 National Quality Strategy domains (NQS).
There are 6 NQS domains to choose from:
- Patient and Family Engagement
- Patient Safety
- Care Coordination
- Population and Public Health
- Efficient Use of Healthcare Resources
- Clinical Processes
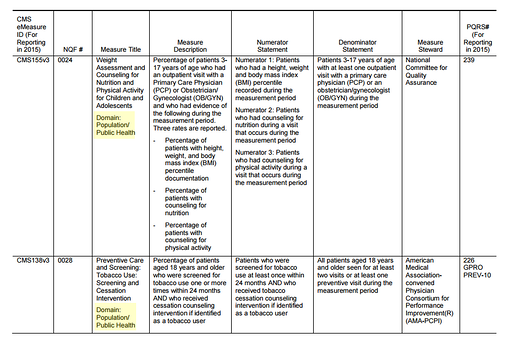
The CMS has provided all possible measurements you can use to fulfill the eCQM requirement, and you can find the domains in each measure title as highlighted below.
Your CQM output must come directly from your EHR software. If you implement a new EHR during your reporting period, the CMS will allow EPs using a 2014 certified system to submit CQMs as an additive number from the two different certified systems as long as you're using the same 9 certified CQMs across both certified systems.
QRDA
Finally, it’s time to report your information through the use of QRDA. QRDA (Quality Reporting Document Architecture) is a standard document format for the exchange of eCQM data. These reports contain data extracted from EHRs and are used for the exchange of eCQM data between systems for quality measurement and reporting initiatives. To streamline implementations, QRDA makes use of CDA templates, which are business rules for representing clinical data consistently.
We are always on the lookout for regulation changes to ensure that ODs get the information they need to be compliant with the Meaningful Use program. Subscribe to our blog to get the latest information!